Transforming Vision Care Across Multiple Markets
Perfecting the sense of sight with LIRIC® — a safe, non-invasive laser technology enabling breakthroughs in contact lenses, myopia control, intraocular lenses, and laser vision correction.
R&D Investment
$80M
Backed by leading investors & partners
Market Opportunity
4 Multi-Billion
Ophthalmic segments targeted
Core Technology
LIRIC Platform
Protected by >90 patents across >30 families
One Platform. Multiple Solutions.
Clerio is a development-stage medical device company tackling huge unmet needs. Our platform addresses critical gaps in vision care that affect hundreds of millions of patients worldwide.
Contact Lenses
Next-gen multifocal optics for aging eyes, delivering superior clarity without compromise.
Learn more →Myopia Control
Protecting children's vision health with therapeutic lenses designed to slow progression.
Learn more →Intraocular Lenses
Non-invasive post-cataract optimization to perfect vision after surgery.
Learn more →Laser Correction
The next revolution: Incisionless corneal refractive correction.
Learn more →Partner in the Next Revolution of Vision Care
One core technology targeting four multi-billion-dollar ophthalmic segments. Join us in shaping the future.
About Clerio Vision
Pioneering non-invasive laser technology to perfect vision and improve quality of life on a global scale.
Our Mission
We are dedicated to providing a wide array of solutions to millions of patients who need better options for their vision correction. Our goal is not just to treat vision errors, but to fundamentally improve the human experience through better sight.
Company Background
Clerio Vision was founded out of Nobel Prize-winning (2018) research at the University of Rochester, a world leader in optics, in collaboration with the renowned Center for Visual Sciences. Developed by pioneers in femtosecond laser physics together with experts in ophthalmology, our journey began as a research collaboration in 2003. Today, we translate deep scientific roots into commercial innovation.
"Clerio's femtosecond laser platform uniquely addresses critical unmet needs across four major ophthalmic segments. This is something few startups or incumbents can claim."
– Arunas Chesonis, Managing Partner and Co-Founder, Safar Partners

Clinical Credibility
First in-human LIRIC trials successfully completed. >80 US Patents held.
LIRIC Technology
Laser Induced Refractive Index Change
What is LIRIC?
LIRIC is a revolutionary laser technique that changes the refractive index of materials, such as hydrogels or corneal tissue, without removing any material. Using ultra-short laser pulses, we "write" a new optical prescription directly inside a lens or the eye by altering the material's density at a molecular level.
The Platform Advantage
This single unifying technology powers our entire product suite. Whether manufacturing advanced contact lenses, adjusting implanted IOLs, or correcting vision in the cornea, the underlying physics remains the same.
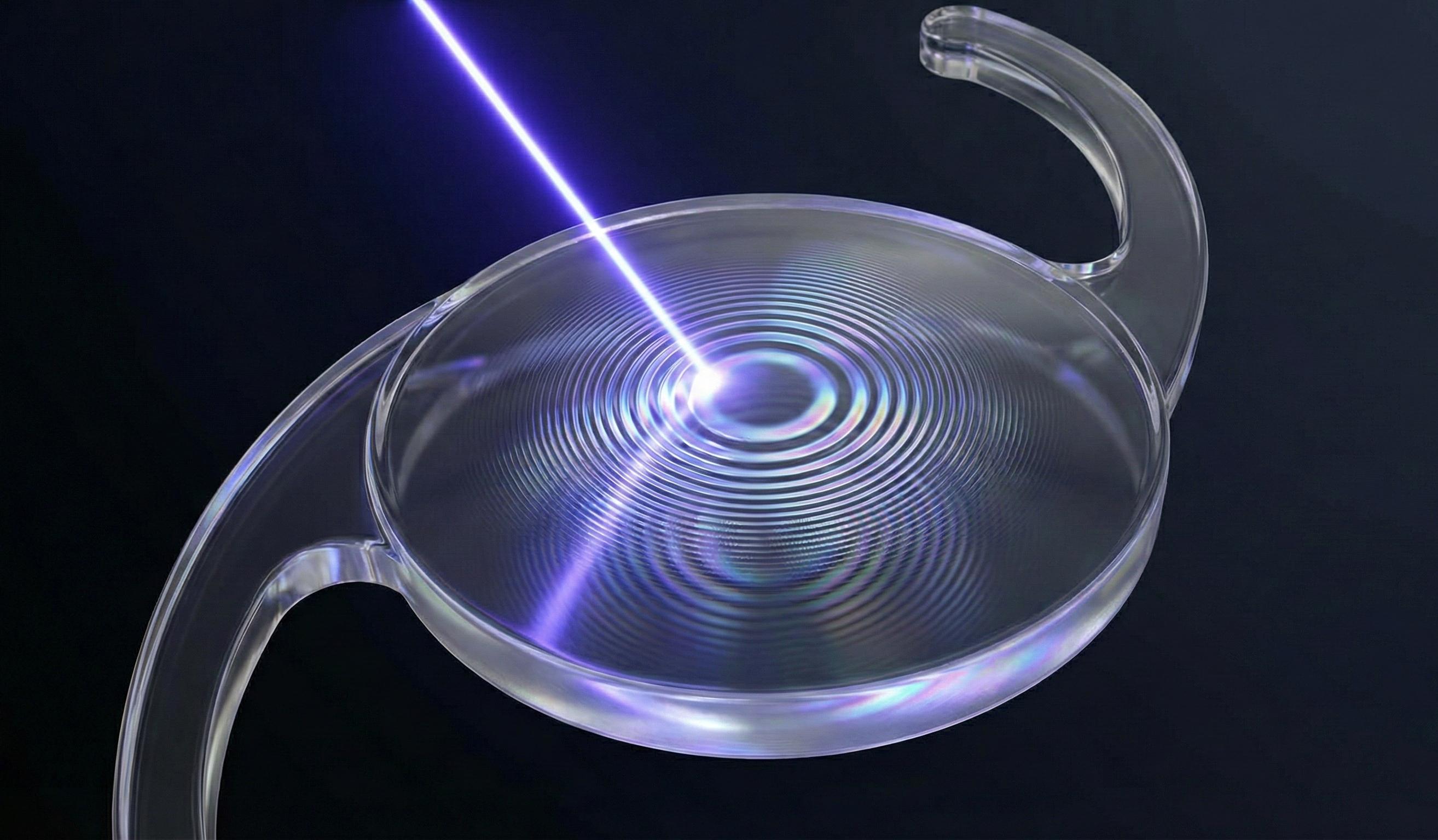
No cutting and no shape change. Pure optical change.
Extreme Precision
Excellent visual acuity and quality from near to far.
Unique Optics
Advanced GRIN optics for presbyopia and myopia management.
Optimization
Ability to adjust optics following surgery.
Repeatable
Corrections can be updated or adjusted as vision changes.
Application Areas
Addressing unmet needs across the continuum of care.
Advanced Multifocal Contact Lenses
Presbyopia affects nearly everyone over 45 or about 1.8 billion people. Current multifocals often compromise visual acuity, visual quality as well as causing halos and glare.
The Clerio Solution
LIRIC-enabled lenses feature computer-generated micro-patterns written inside the lens material. This offers superior visual quality, reduced halos, and sharp vision at all distances.
- Proprietary diffractive structures impossible to mold.
- Customizable zones for precision optics.
- Targeting the $2.5B global presbyopia market.


Next-Gen Myopia Control
Myopia is a global epidemic, projected to affect 50% of the world by 2050. It increases risks of severe eye disease. No current solution stops it completely.
The Clerio Solution
We are developing soft contacts optimized to slow myopia progression more effectively. LIRIC allows for tailored defocus patterns that induce stronger myopia-slowing signals while maintaining comfort for children.
Post-Implant IOL Adjustment
~30% of cataract patients have residual refractive error after surgery. Most are stuck with it or need glasses.
The Clerio Solution
A device that uses LIRIC to "write" corrective optics into an IOL after it has been implanted inside the eye.
- Non-invasive, in-office procedure.
- Can upgrade a standard monofocal lens to a multifocal lens.
- Works with standard IOL materials.


Incisionless Vision Correction
Conventional LASIK involves cutting a corneal flap and ablating tissue, which deters many patients due to fear and dry-eye risks.
The Clerio Solution
LIRIC Vision Correction alters the cornea's refractive index without a single cut. No flap. No tissue removal. No ablation.
- Significantly reduces dry eye risk (no nerves severed).
- Preserves corneal structural integrity.
- Addresses the "fear factor" of surgery.
Our Team
Deep scientific expertise combined with industry veteran leadership.
Leadership Team

J. Mikael Totterman
Chairman
View Profile →
Alex Zapesochny
CEO
View Profile →
Anna Schreyer
EVP of Development Operations
View Profile →
Alissa Seidman
EVP of Finance
View Profile →Technical Leadership Team

Len Zheleznyak, PhD
EVP of Vision Science
View Profile →
Filipp Ignatovich, PhD
VP of Advanced Ophthalmic Technologies
View Profile →
Lisen Xu, PhD
VP of Applied Research
View Profile →
Wayne Knox, PhD
Chief Science Officer
View Profile →
Michele Lagana, OD
Principal Investigator
View Profile →
Ian Cox, OD, PhD
Director, Scientific Clinical Affairs
View Profile →Outside Board Members

Arunas Chesonis
Board Member
View Profile →
Robert P. Cochran
Board Member
View Profile →
Brian Model
Board Member
View Profile →
David E. Williams
Board Member
View Profile →News & Media
Latest updates, press releases, and industry coverage.

Clerio Vision Announces Successful Completion of Financing Round
Investment to accelerate development of the LIRIC contact lens platform.
Read Full ReleaseClerio Vision to Unveil Breakthrough LIRIC Contact Lens Data and New Myopia Research at 2026 Wavefront Congress
Executive VP of Vision Science, Dr. Len Zheleznyak, to present performance data on the world's only diffractive multifocal soft contact lens and new findings on the "Optical Signatures" of myopia.
Clerio Vision Granted U.S. Patent for Novel "Optical Signature" Technology to Control Myopia Progression
New intellectual property secures method for manipulating peripheral blur orientation, a key signal for regulating eye growth, paving the way for upcoming clinical trials.
Clerio Vision Executive Co-Authors Breakthrough Study Identifying Potential New "Optical Signature" for Myopia Control
Pivotal new study identifies a critical difference in how myopic and emmetropic eyes process peripheral light.
Clerio Vision Executives Present New Data on "Optical Triggers" of Myopia at Major China Symposium
New research presented in Hangzhou identifies peripheral retinal defocus as a key driver of eye growth, positioning LIRIC technology as a unique solution for halting myopia progression.
Clerio Vision Executive Delivers Keynote at VPO 2024
Correcting Chromatic Aberration Unlocks New Frontier in Myopia Control and Visual Performance.
Clerio Vision Announces Successful Completion of Financing Round

Rochester, NY — Clerio Vision, Inc., a developer of next-generation vision correction platforms, today announced the successful closing of its financing round.
The round was led by Safar Partners, with participation from existing investors including Hegemon Capital. The influx of capital will be primarily dedicated to accelerating the clinical development of the company’s proprietary LIRIC technology.
"This funding represents a significant vote of confidence in our team and our technology," said Alex Zapesochny, CEO of Clerio Vision. "We are now well-positioned to complete our next set of multifocal contact lens studies and bring this transformative, non-invasive technology to patients worldwide."
Unlike traditional multifocal contact lenses, LIRIC uses a femtosecond laser to inscribe diffractive optics into the contact lens to provide improved visual acuity and quality across all distances.
Contact:
Alissa Seidman
aseidman@cleriovision.com
Clerio Vision to Unveil Breakthrough LIRIC Contact Lens Data and New Myopia Research at 2026 Wavefront Congress
Executive VP of Vision Science, Dr. Len Zheleznyak, to present performance data on the world's only diffractive multifocal soft contact lens and new findings on the "Optical Signatures" of myopia
ROCHESTER, NY – February 11, 2026 – Clerio Vision, Inc., a developer of next-generation laser-based vision correction platforms, today announced that it will be delivering two key presentations at the upcoming annual Wavefront Congress, to be held in Miami, Florida, beginning February 27th.
Dr. Len Zheleznyak, Executive Vice President of Vision Science at Clerio Vision, will present new data highlighting the company's dual innovations in presbyopia and myopia management. The presentations will showcase how Clerio's proprietary LIRIC (Laser Induced Refractive Index Change) technology is being used to create optical designs that were previously impossible to manufacture in contact lenses.
Presentation Highlights
The World's Only Diffractive Multifocal Contact Lens
Dr. Zheleznyak will report visual performance data for a groundbreaking new soft contact lens design enabled by LIRIC. Unlike traditional molding or lathing, LIRIC allows for the embedding of a diffractive wavefront directly within the contact lens material. This capability has produced an Extended Depth of Focus (EDOF) design that offers superior visual quality across distances, marking the first time a diffractive multifocal optic has been successfully deployed within a soft contact lens format.
New "Optical Signatures" in Myopic Children
In a second session, Dr. Zheleznyak will discuss findings from a recent paper published in the Journal of the Optical Society of America A1. The study, a multi-national collaboration involving Aier Eye Hospital (China), the University of Rochester (USA), Wroclaw University of Science and Technology (Poland), and the University of Murcia (Spain), examined retinal image quality in Chinese children. The researchers identified a distinct trend: myopic children tend to exhibit an anti-radially elongated peripheral blur, a marked difference from the blur patterns found in emmetropic (non-myopic) children. This finding supports the hypothesis that peripheral blur orientation is a critical signal for eye growth.
"The Wavefront Congress is the premier forum for the world's leading vision scientists," said Alex Zapesochny, CEO of Clerio Vision. "We are thrilled to share our latest clinical and research work with this community. From defining the optical triggers of myopia to creating the world's first diffractive soft contact lens, our team is demonstrating the unique power of the LIRIC platform to solve complex vision challenges."
About Clerio Vision
Clerio Vision is developing a breakthrough laser technology platform to treat vision issues. The company's proprietary LIRIC technology modifies the refractive index of existing lens materials, enabling the non-invasive correction of refractive errors and the creation of advanced optical designs for presbyopia, myopia, and higher-order aberration correction.
References
1 Winter, S., Liu, C., Lin, Z., Artal, P., Lan, W., and Zheleznyak, L., 2025. Optical quality and anisotropy across the retina of Chinese children's eyes. Journal of the Optical Society of America A, 43(1), pp.77-85.
Contact:
Alissa Seidman
aseidman@cleriovision.com
Clerio Vision Executive Co-Authors Breakthrough Study Identifying Potential New "Optical Signature" for Myopia Control
Clerio Vision, Inc., a developer of next-generation vision correction products, today announced the publication of a pivotal new study co-authored by Len Zheleznyak, EVP of Vision Science at Clerio Vision.
The keynote, titled "Chromatic Aberration and Eye Growth," presented a novel framework for understanding how the eye focuses different wavelengths of light, known as Longitudinal Chromatic Aberration (LCA), and how correcting this aberration can fundamentally transform contact lens performance.
The "Stop" Sign for Myopia
Dr. Zheleznyak's presentation highlighted a critical link between LCA and the progression of myopia (nearsightedness). While traditional theories focus on refractive blur, research (Gawne, Schaeffel) suggests that the eye uses the separation of colors (chromatic cues) to determine its growth direction. By precisely correcting LCA, a contact lens can provide a distinct "stop" signal to the eye, potentially halting the axial elongation that defines myopia.
"For decades, we have treated LCA as a minor optical imperfection," said Dr. Zheleznyak. "Previous research indicates it is actually a primary driver of eye growth. By correcting LCA, we are not just sharpening the image; we are changing the biological instructions the eye receives."
Enhancing Visual Acuity for All Patients
Beyond pediatric applications, the keynote demonstrated that LCA correction offers significant benefits for the general adult population. Conventional single-vision contact lenses often leave residual chromatic blur, which limits the "crispness" of vision. Zheleznyak presented data (Roorda / UC Berkley and Nankivil / JnJ) showing that correcting LCA of the eye results in measurable improvements in visual acuity and contrast sensitivity, offering a "high-definition" visual experience unavailable in current standard soft lenses.
LIRIC: The Enabling Technology
Correcting LCA in a soft contact lens has historically been impossible with traditional molding or lathe-cutting manufacturing methods. Clerio Vision’s LIRIC technology is the only platform capable of writing the necessary diffractive-like optical patterns into a lens material to correct LCA.
"The optical profiles required to correct Longitudinal Chromatic Aberration are incredibly complex," said Alex Zapesochny, CEO of Clerio Vision. "You cannot simply mold them. You need to write them directly into the material with extreme precision. LIRIC allows us to impart these advanced optical corrections into standard contact lenses, opening the door to a new generation of products that both correct vision better than ever before and actively manage eye health."
About Clerio Vision
Clerio Vision is developing a breakthrough laser technology platform to treat vision issues. The company’s proprietary LIRIC technology modifies the refractive index of existing lens materials, enabling the non-invasive correction of refractive errors and the creation of advanced optical designs for presbyopia, myopia, and higher-order aberration correction.
Contact:
Alissa Seidman
aseidman@cleriovision.com
Clerio Vision Granted U.S. Patent for Novel "Optical Signature" Technology to Control Myopia Progression
New intellectual property secures method for manipulating peripheral blur orientation, a key signal for regulating eye growth, paving the way for upcoming clinical trials.
ROCHESTER, NY – January 5, 2026 – Clerio Vision, Inc., a developer of next-generation laser-based vision correction platforms, today announced the issuance of U.S. Patent No. 12,443,053 B2, titled "Myopia Control Treatments". The patent, awarded to inventors Len Zheleznyak, PhD, and Gustavo Gandara-Montano, PhD, covers a breakthrough lens design capable of modifying the orientation of light spread in the peripheral retina.
This intellectual property milestone supports Clerio Vision's developing portfolio of myopia control interventions. The patented technology addresses a critical "Optical Signature" identified by Clerio researchers: the specific orientation of peripheral blur.
The "Shape" of Focus Matters
Traditional myopia treatments often focus on the amount of peripheral defocus (blur). However, Clerio Vision's research indicates that the orientation of that blur, known as Optical Anisotropy, may be a more potent signal for the eye.
"Our research suggests that the eye is sensitive to the shape of the focal point in the peripheral retina," said Len Zheleznyak, EVP of Vision Science at Clerio Vision and co-inventor of the technology. "We have found that 'anti-radial' blur is endemic to myopic eyes. Switching that blur orientation to radial may be protective against myopia progression, based on choroidal thickness studies. This patent covers optical designs needed to flip peripheral blur orientation from 'grow' to 'stop' using contact lenses".
LIRIC: Precision Where It Matters
Creating these highly specific, asymmetric optical patterns is challenging with conventional contact lens molding. Clerio Vision's proprietary LIRIC technology is uniquely capable of "writing" these complex optical anisotropy profiles directly into the lens material with micrometer-level precision. This allows for the creation of a "stop signal" that is customized to the eye's natural optics.
Next Steps: Clinical Validation
With this key patent secured, Clerio Vision is moving forward with the design of additional clinical trials to validate the efficacy of these novel optical profiles in pediatric populations. The company aims to demonstrate that manipulating the orientation of peripheral blur can significantly slow the progression of myopia in children.
"Securing this patent is a pivotal step in our roadmap," said Alex Zapesochny, CEO of Clerio Vision. "It protects the fundamental mechanism of action behind our next-generation myopia control designs and allows us to proceed confidently into the clinical trial phase. We are excited to translate this scientific discovery into a therapeutic reality for millions of myopic children."
About Clerio Vision
Clerio Vision is developing a breakthrough laser technology platform to treat vision issues. The company's proprietary LIRIC technology modifies the refractive index of existing lens materials, enabling the non-invasive correction of refractive errors and the creation of advanced optical designs for presbyopia, myopia, and higher-order aberration correction.
Contact:
Alissa Seidman
aseidman@cleriovision.com
Clerio Vision Executives Present New Data on "Optical Triggers" of Myopia at Major China Symposium
New research presented in Hangzhou identifies peripheral retinal defocus as a key driver of eye growth, positioning LIRIC technology as a unique solution for halting myopia progression.
Clerio Vision, Inc., a developer of next-generation laser-based vision correction platforms, today announced that its leadership team presented groundbreaking new findings at the New Technology Symposium on Retinal Peripheral Vision held this month in Hangzhou, China.
Alex Zapesochny, CEO, and Len Zheleznyak, PhD, EVP of Vision Science, shared data from their presentation titled "The Role of Optical Cues in Myopia," which details how specific light signals in the peripheral retina act as "traffic lights" for eye growth, signaling the eye to either "STOP" or "GROW".
With global myopia prevalence projected to reach 50% of the world's population by 2050, identifying the root biological drivers of the condition has become urgent. The data presented by Clerio Vision highlights that the eye is the only organ whose growth is directly guided by photons, specifically through optical cues such as light levels, spectrum, and optical aberrations found in the peripheral visual field.
Decoding the "Optical Code" of Eye Growth
The presentation revealed that the interaction between the eye's natural Longitudinal Chromatic Aberration (LCA) and peripheral wavefront aberrations creates a distinct "optical signature" that the retina uses to determine whether to elongate.
"Our research demonstrates that the orientation and color of blur in the peripheral retina differ fundamentally between myopic and emmetropic (normal) eyes," said Dr. Zheleznyak. "We found that specific optical cues, such as the orientation of peripheral astigmatism, effectively tell the eye whether an image is focused in front of or behind the retina. By deciphering these cues, we can understand why near-work environments, which often present anti-radial blur patterns, may trigger excessive eye growth".
LIRIC: Writing the Cure into the Lens
While traditional contact lens manufacturing is limited in its ability to manipulate these complex peripheral signals, Clerio's proprietary LIRIC technology offers a novel solution. LIRIC can "write" precise, diffractive-like optical patterns directly into the contact lens material to mimic the protective optical cues found in emmetropic eyes, without degrading central vision quality.
"China is at the forefront of the myopia epidemic, with prevalence rates in urban centers like Hong Kong and Singapore having skyrocketed over the last few decades," said Alex Zapesochny, CEO of Clerio Vision. "It was an honor to share our findings in Hangzhou. We believe LIRIC is uniquely suited to address this challenge because it allows us to imprint the exact optical 'stop signal' the eye needs directly into a contact lens, offering a potential functional cure for myopia progression."
About Clerio Vision
Clerio Vision is developing a breakthrough laser technology platform to treat vision issues. The company's proprietary LIRIC technology modifies the refractive index of existing lens materials, enabling the non-invasive correction of refractive errors and the creation of advanced optical designs for presbyopia and myopia.
Contact:
Alissa Seidman
aseidman@cleriovision.com
Clerio Vision Executive Delivers Keynote at VPO 2024: Correcting Chromatic Aberration Unlocks New Frontier in Myopia Control and Visual Performance
Clerio Vision, Inc., a developer of next-generation laser-based vision correction platforms, today announced that Len Zheleznyak, PhD, Executive Vice President of Vision Science, delivered a keynote lecture at the prestigious Visual and Physiological Optics (VPO) Conference held this week in Wroclaw, Poland.
The keynote, titled "Chromatic Aberration and Eye Growth," presented a novel framework for understanding how the eye focuses different wavelengths of light, known as Longitudinal Chromatic Aberration (LCA), and how correcting this aberration can fundamentally transform contact lens performance.
The "Stop" Sign for Myopia
Dr. Zheleznyak's presentation highlighted a critical link between LCA and the progression of myopia (nearsightedness). While traditional theories focus on refractive blur, research (Gawne, Schaeffel) suggests that the eye uses the separation of colors (chromatic cues) to determine its growth direction. By precisely correcting LCA, a contact lens can provide a distinct "stop" signal to the eye, potentially halting the axial elongation that defines myopia.
"For decades, we have treated LCA as a minor optical imperfection," said Dr. Zheleznyak. "Our research indicates it is actually a primary driver of eye growth. By correcting LCA, we are not just sharpening the image; we are changing the biological instructions the eye receives."
Enhancing Visual Acuity for All Patients
Beyond pediatric applications, the keynote demonstrated that LCA correction offers significant benefits for the general adult population. Conventional single-vision contact lenses often leave residual chromatic blur, which limits the "crispness" of vision. Zheleznyak presented data (Roorda, Nankivil) showing that correcting LCA of the eye results in measurable improvements in visual acuity and contrast sensitivity, offering a "high-definition" visual experience unavailable in current standard soft lenses.
LIRIC: The Enabling Technology
Correcting LCA in a soft contact lens has historically been impossible with traditional molding or lathe-cutting manufacturing methods. Clerio Vision’s LIRIC technology is the only platform capable of writing the necessary diffractive-like optical patterns into a lens material to correct LCA.
"The optical profiles required to correct Longitudinal Chromatic Aberration are incredibly complex," said Alex Zapesochny, CEO of Clerio Vision. "You cannot simply mold them. You need to write them directly into the material with extreme precision. LIRIC allows us to impart these advanced optical corrections into standard contact lenses, opening the door to a new generation of products that both correct vision better than ever before and actively manage eye health."
About Clerio Vision
Clerio Vision is developing a breakthrough laser technology platform to treat vision issues. The company’s proprietary LIRIC technology modifies the refractive index of existing lens materials, enabling the non-invasive correction of refractive errors and the creation of advanced optical designs for presbyopia, myopia, and higher-order aberration correction.
Contact:
Alissa Seidman
aseidman@cleriovision.com
Get in Touch
Whether you are an investor, a potential partner, or a job seeker, we'd love to hear from you.
Headquarters
Clerio Vision Inc.
1892 South Winton Road
Rochester, NY 14618
Manufacturing
205 Summit Point Dr
Henrietta, NY 14467
info@cleriovision.com
Phone
+1 (585) 565-4500
Headquarters
Manufacturing
Privacy Policy
Clerio Vision, Inc.
Effective Date: January 7, 2026
Last Modified: February 2, 2026
1. Introduction and Scope
Clerio Vision, Inc. ("Clerio Vision," "we," "us," or "our") respects your privacy and is committed to protecting your personal data. This Privacy Policy outlines our practices regarding the collection, use, and disclosure of information we collect from you or that you provide to us when you visit our website www.cleriovision.com (the "Site"), contact us regarding our technology, inquire about clinical trials, or apply for employment.
Important Note Regarding Clinical Trials: This Privacy Policy applies to the use of our public-facing website. If you are enrolled in a Clerio Vision clinical trial, your personal health information is governed by the specific Informed Consent Form (ICF) and Patient Information Sheet provided to you by the study doctor or clinical site. In the event of a conflict between this Website Privacy Policy and the ICF, the terms of the ICF shall prevail regarding your study data.
2. Information We Collect About You
We collect several types of information from and about users of our Site, depending on how you interact with us.
2.1 Information You Provide to Us
-
Identity and Contact Data: When you fill out forms on our Site (such as "Contact Us" or "Investor Inquiries"), we collect your name, email address, postal address, phone number, and professional affiliation.
-
Clinical Interest Data: If you contact us to express interest in participating in a clinical trial (e.g., for myopia or presbyopia), you may voluntarily provide limited information about your age, location, and general vision status. We urge you not to submit sensitive medical records, detailed health histories, or other Protected Health Information (PHI) through the general contact forms on this Site.
-
Employment Data: If you apply for a position via our "Careers" page, we collect your curriculum vitae (CV), employment history, and educational background.
2.2 Information We Collect Automatically
As you navigate through and interact with our Site, we may use automatic data collection technologies to collect certain information about your equipment, browsing actions, and patterns:
-
Device Data: Internet Protocol (IP) address, operating system, browser type, and screen resolution.
-
Usage Data: Details of your visits to our Site, including traffic data, location data, logs, and other communication data and the resources that you access and use.
3. How We Use Your Information
We use information that we collect about you or that you provide to us, including any personal information:
-
To Provide Information: To present our Site and its contents to you and to respond to your inquiries regarding our LIRIC® technology.
-
Clinical Trial Recruitment: To evaluate your potential eligibility for our clinical studies and, with your consent, to connect you with a clinical research site in your region (e.g., in the US or Europe).
-
Business Administration: To manage our relationship with investors and partners, including communicating corporate updates and financial information where appropriate.
-
Compliance and Security: To comply with legal obligations (such as FDA reporting requirements or the New York SHIELD Act), to enforce our Terms of Use, and to protect the security of our Site.
4. Legal Basis for Processing (EEA/UK Users)
If you are located in the European Economic Area (EEA) or the United Kingdom (UK), our legal basis for collecting and using the personal information described above will depend on the personal information concerned and the specific context in which we collect it.
| Processing Activity | Legal Basis (GDPR/UK GDPR) |
|---|---|
| Website Operation & Security | Legitimate Interests: It is in our legitimate interest to monitor and improve our website security and functionality. |
| Responding to General Inquiries | Legitimate Interests: It is in our legitimate interest to respond to your questions and manage our business relationship. |
| Clinical Trial Recruitment | Consent: We process health-related inquiries based on your explicit consent. You may withdraw this consent at any time. |
| Adverse Event Reporting | Legal Obligation: We are required by law to report safety data to health authorities (e.g., FDA, EMA). |
5. Disclosure of Your Information
We do not sell your personal information. We may disclose aggregated information about our users, and information that does not identify any individual, without restriction. We may disclose personal information that we collect or you provide as described in this privacy policy:
-
Service Providers: To contractors, service providers, and other third parties we use to support our business (e.g., cloud hosting providers, applicant tracking systems) who are bound by contractual obligations to keep personal information confidential and use it only for the purposes for which we disclose it to them.
-
Clinical Research Partners: If you inquire about a study, we may share your contact details with the specific Clinical Research Organization (CRO) or investigative site (e.g., university hospital) managing the trial in your geographic area.
-
Regulatory Authorities: To the FDA, EMA, or other government regulatory authorities as required by law to report adverse events or product safety information.
-
Business Transfers: To a buyer or other successor in the event of a merger, divestiture, restructuring, reorganization, dissolution, or other sale or transfer of some or all of Clerio Vision's assets.
6. International Data Transfers
Clerio Vision is headquartered in the United States. If you access the Site from the European Union, United Kingdom, or other regions with laws governing data collection and use, please note that you are agreeing to the transfer of your personal information to the United States. The United States may have data protection laws that are different from the laws of your country.
Transfer Mechanisms: When we transfer personal data from the EEA or UK to the US, we rely on adequate transfer mechanisms, such as the European Commission's Standard Contractual Clauses (SCCs), to ensure your data is afforded a similar degree of protection.
7. Data Security
We have implemented measures designed to secure your personal information from accidental loss and from unauthorized access, use, alteration, and disclosure.
New York SHIELD Act: In compliance with the New York SHIELD Act, we maintain a comprehensive information security program that includes reasonable administrative, technical, and physical safeguards to protect the private information of our users.
Disclaimer: Unfortunately, the transmission of information via the internet is not completely secure. Although we do our best to protect your personal information, we cannot guarantee the security of your personal information transmitted to our Site.
8. Data Retention
We retain your personal information only for as long as is necessary to fulfill the purposes for which we collected it, including for the purposes of satisfying any legal, accounting, or reporting requirements.
Clinical Inquiries: Personal data related to clinical trial inquiries is retained for the duration of the recruitment period and a reasonable period thereafter to ensure trial integrity, unless you request deletion sooner.
9. Your Rights
Depending on your jurisdiction, you may have the following rights regarding your personal information:
The right to request access to your personal data.
The right to request correction of the personal data that we hold about you.
The right to request erasure of your personal data ("Right to be Forgotten").
The right to request restriction of processing of your personal data.
The right to request the transfer of your personal data to another party.
To exercise any of these rights, please contact our Privacy Officer:
Alissa Seidman
Email: aseidman@cleriovision.com
Clerio Vision, Inc.
1892 South Winton Road, Suite 150
Rochester, NY 14618, USA
10. Children's Privacy
Our Site is not intended for children under 16 years of age. We do not knowingly collect personal information from children under 16. If you are a parent or guardian and are interested in our myopia control technology for your child, please contact us directly.
If we learn we have collected or received personal information from a child under 16 without verification of parental consent, we will delete that information.
11. Changes to Our Privacy Policy
It is our policy to post any changes we make to our privacy policy on this page. The date the privacy policy was last revised is identified at the top of the page. You are responsible for periodically visiting our Website and this privacy policy to check for any changes.
Terms of Use
Clerio Vision, Inc.
Effective Date: January 7, 2026
Last Updated: February 2, 2026
1. Acceptance of the Terms of Use
Welcome to the website of Clerio Vision, Inc. ("Company," "we," or "us"). The following terms and conditions (these "Terms of Use"), govern your access to and use of www.cleriovision.com, including any content, functionality, and services offered on or through the website (the "Website").
Please read the Terms of Use carefully before you start to use the Website. By using the Website, you accept and agree to be bound and abide by these Terms of Use and our . If you do not want to agree to these Terms of Use, you must not access or use the Website.
2. Medical Disclaimer – No Medical Advice
Important Notice
THE INFORMATION PROVIDED ON THIS WEBSITE IS FOR GENERAL INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. IT IS NOT INTENDED TO AMOUNT TO MEDICAL ADVICE ON WHICH YOU SHOULD RELY.
-
No Doctor-Patient Relationship: Use of this Website, or transmission of information via the "Contact Us" forms, does not establish a doctor-patient relationship between you and Clerio Vision.
-
Consult a Professional: You must obtain professional or specialist advice from a qualified healthcare provider before taking, or refraining from, any action on the basis of the content on our Website. The LIRIC™ technology described herein is under development; there are no guarantees that it will be available for your specific condition.
-
Emergency: If you think you may have a medical emergency, call your doctor or emergency services immediately.
3. Regulatory Status – Investigational Device Caution
Clerio Vision is a development-stage medical device company. The products and technologies described on this Website, including the LIRIC platform and associated contact lens or corneal applications, are investigational devices and have not yet received marketing approval or clearance from the U.S. Food and Drug Administration (FDA) or other regulatory bodies.
CAUTION – Investigational device. Limited by Federal (or United States) law to investigational use. This device is not available for sale or commercial distribution in the United States.
The regulatory status of our products may vary by country. Nothing on this Website should be construed as a promotion or solicitation for any product or for the use of any product that is not authorized by the laws and regulations of your country of residence.
4. Intellectual Property Rights
The Website and its entire contents, features, and functionality (including but not limited to all information, software, text, displays, images, video, and audio, and the design, selection, and arrangement thereof) are owned by the Company, its licensors, or other providers of such material and are protected by United States and international copyright, trademark, patent, trade secret, and other intellectual property or proprietary rights laws.
Clerio Vision's technology is protected by a robust portfolio of issued and pending patents related to Laser Induced Refractive Index Change. This Website serves as constructive notice under 35 U.S.C. § 287(a).
The Clerio Vision name, the LIRIC logo, and all related names, logos, product and service names, designs, and slogans are trademarks of the Company. You must not use such marks without the prior written permission of the Company.
5. Forward-Looking Statements
This Website contains "forward-looking statements" regarding our future operating and financial performance, clinical trial timelines, and regulatory pathways. These statements are based on our current expectations and are subject to inherent uncertainties and risks that could cause actual results to differ materially.
-
Nature of Risk: Risks include, but are not limited to: the inherent uncertainty of clinical research (e.g., trials may fail to demonstrate safety or efficacy); the timing and outcome of regulatory reviews by the FDA and other authorities; our ability to manufacture products in compliance with Quality System Regulations; and our ability to raise additional capital.
-
No Guarantee: There is no guarantee that the LIRIC technology will ever be approved for commercial sale. The Company undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future developments, or otherwise, except as required by law.
6. User Conduct and Unsolicited Ideas
-
Prohibited Uses: You agree not to use the Website to transmit any material that is defamatory, obscene, compliant, or infringes on the intellectual property rights of others.
-
Unsolicited Ideas: Clerio Vision does not accept or consider unsolicited ideas, including ideas for new advertising campaigns, new promotions, new products, or technologies. If you send us such information, you agree that your submission will not be considered confidential or proprietary, and Clerio Vision shall be free to use such information for any purpose without compensation to you.
7. Disclaimer of Warranties
YOUR USE OF THE WEBSITE IS AT YOUR OWN RISK. THE WEBSITE IS PROVIDED ON AN "AS IS" AND "AS AVAILABLE" BASIS, WITHOUT ANY WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED. THE COMPANY DISCLAIMS ALL WARRANTIES OF MERCHANTABILITY, NON-INFRINGEMENT, AND FITNESS FOR A PARTICULAR PURPOSE.
8. Limitation on Liability
TO THE FULLEST EXTENT PROVIDED BY LAW, IN NO EVENT WILL CLERIO VISION, ITS AFFILIATES, OR THEIR LICENSORS BE LIABLE FOR DAMAGES OF ANY KIND, UNDER ANY LEGAL THEORY, ARISING OUT OF OR IN CONNECTION WITH YOUR USE, OR INABILITY TO USE, THE WEBSITE, INCLUDING ANY DIRECT, INDIRECT, SPECIAL, INCIDENTAL, CONSEQUENTIAL, OR PUNITIVE DAMAGES.
9. Governing Law and Jurisdiction
All matters relating to the Website and these Terms of Use, and any dispute or claim arising therefrom or related thereto, shall be governed by and construed in accordance with the internal laws of the State of New York without giving effect to any choice or conflict of law provision or rule.
Any legal suit, action, or proceeding arising out of, or related to, these Terms of Use shall be instituted exclusively in the federal courts of the United States or the courts of the State of New York, in each case located in the County of Monroe (Rochester).
Our Investors
Backed by leading venture capital partners committed to transforming vision care.
Clerio Vision is proud to be supported by a distinguished group of venture capital partners who share our vision of revolutionizing eye care through innovative LIRIC technology.
Safar Partners
Investor
A technology venture fund with over $1B under management, investing in companies from leading universities including MIT, Harvard, and the University of Rochester. Focus areas include cleantech, AI/IT, robotics, and life sciences.
Visit Website
Topmark Partners
Investor
A venture capital firm focused on early-stage investments in innovative companies with breakthrough technologies and strong growth potential.
Visit Website
Armory Square Ventures
Investor
A seed and early-stage venture capital fund investing in high-growth technology companies across the Northeast and beyond.
Visit Website
Hegemon Capital
Investor
A private investment firm focused on providing growth capital to innovative companies with disruptive technologies and strong market positioning.
Visit Website
ATMA Capital
Investor
A venture capital firm dedicated to backing visionary entrepreneurs building transformative companies in healthcare, technology, and life sciences.
Visit Website
Proxima Ventures
Investor
A venture capital firm investing in early-stage companies with breakthrough innovations and the potential to create significant impact in their respective markets.
Visit WebsiteWhy Investors Choose Clerio Vision
Our unique platform technology and experienced team position us to capture significant value across multiple ophthalmic markets.
R&D Investment
Multi-Billion Dollar Markets
Patents Protecting LIRIC
Interested in Learning More?
For investor inquiries or partnership opportunities, please reach out to our team.